Why Should You Get a Sterile Compounding and Aseptic Certification?
Sterile Compounding & Aseptic Certification: Why It Truly Matters
Quick Summary:
|
Pharmacy is changing fast, and sterile compounding has moved from being a “specialized skill” to an essential requirement in hospitals, infusion centers, and specialty pharmacies. The growing focus on patient safety, compliance, and personalized treatments is driving demand for certified sterile compounding professionals.
According to the U.S. Bureau of Labor Statistics, employment of pharmacy technicians is projected to grow 7% from 2022 to 2032, faster than average for all occupations—driven in large part by the need for sterile compounding in hospitals and infusion centers.
The rise of biologics, personalized IV therapy, and outpatient infusion centers has made sterile compounding one of the most important—and regulated—skills in pharmacy today.
What Sterile Compounding Is (and Why It’s High Stakes)
Sterile compounding involves preparing compounded sterile preparations (CSPs) such as IVs, injections, chemotherapy doses, eye drops, and irrigations. Because these medications bypass the body’s natural defenses, a single error or contaminant can lead to severe infections or worse.
By contrast, non-sterile compounding (USP <795>) covers creams, pills, suspensions, and doesn’t require cleanrooms or aseptic garbing.
The difference? Risk. CSPs demand ISO-classified environments, aseptic technique, and zero-contamination precision.
Who Performs Sterile Compounding?
- Pharmacy Technicians: Often handle day-to-day sterile compounding under pharmacist supervision. In states like Texas, a 40-hour sterile training course and documented competencies are legally required before compounding.
- Pharmacists: Supervise, verify CSPs, lead cleanroom compliance, and, in advanced roles, often pursue Board Certified Sterile Compounding Pharmacist (BCSCP) credentials.
Both roles depend on proper training and ongoing certification to ensure safe, compliant practice.
Why Certification Matters—Really
Credentials in sterile compounding aren’t vanity—they’re a safety net for patients and a springboard for your career. Here’s exactly how certification pays off at the hood and on your résumé:
Benefit | Why It Matters |
Patient Safety | Aseptic precision prevents contamination that could cause life-threatening infections. |
Compliance | Certification proves you’re aligned with 2023 USP <797> updates. |
Career Growth | Opens IV room, oncology, and specialty compounding roles with better pay. |
Employer Confidence | Hospitals prefer CSPT® or BCSCP-certified staff. |
Future-Proofing | Certification ensures your skills stay relevant as regulations evolve. |
USP <797> 2023 – At a Glance
USP <797> received a major overhaul on November 1, 2023, replacing the old risk levels with a category system and tightening competency and monitoring requirements.
Here’s a quick side-by-side so you can see exactly what changed—use it to align SOPs, training cadence, and BUD policies at your site.
Area | Old Standard | New 2023 Revision |
Categories | Low/Med/High risk | Category 1, 2, 3 (Cat 3 allows longer BUDs) |
Competency | Every 6 months | Cat 3 every 3 months (media fill + gloved fingertip) |
Surface Sampling | Not consistent | Monthly (Cat 1/2); Weekly (Cat 3) |
Immediate-use BUD | 1 hour | Up to 4 hours (limited criteria) |
Why it matters: If you’re trained only on “low/medium/high” risk levels, you’re outdated. Certification aligns you with the current standard.
Path to Certification (Techs & Pharmacists)
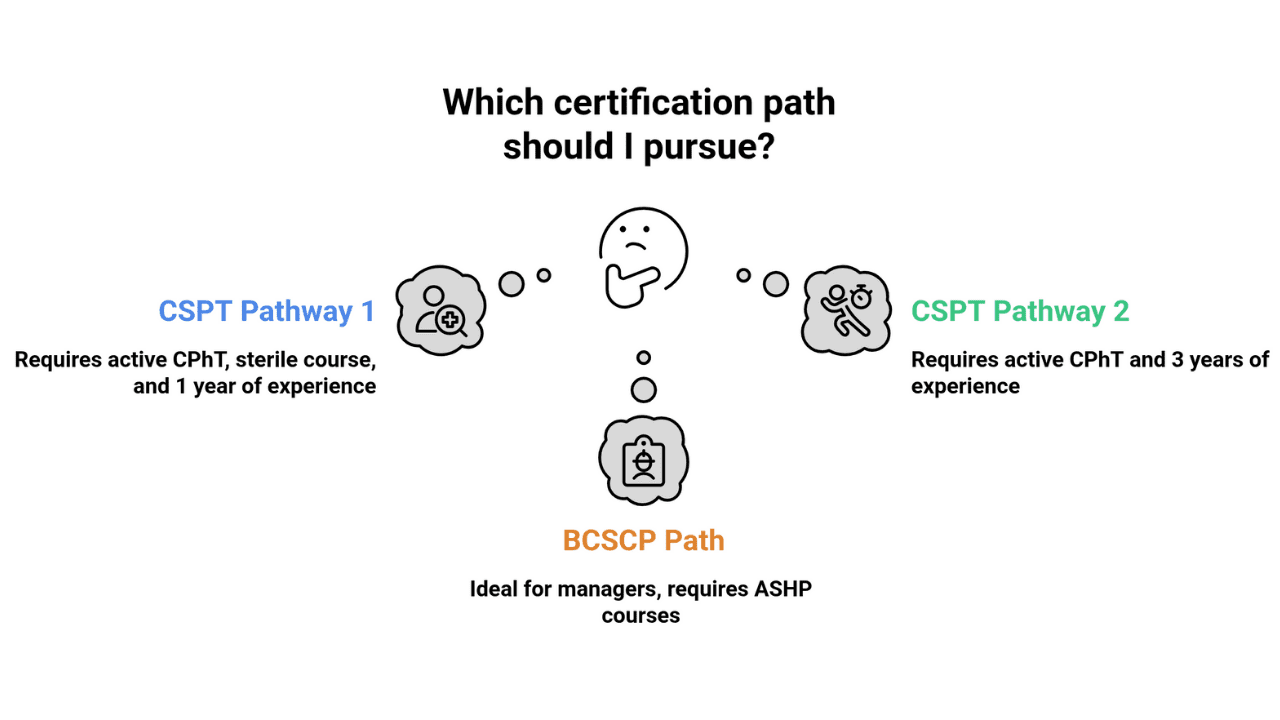
Pharmacy Technicians – CSPT® Path
- Pathway 1: Active CPhT + PTCB-recognized sterile course + 1 year sterile experience
- Pathway 2: Active CPhT + 3 years sterile experience in 8 years
- Exam: 75 questions, 110 minutes
- Renewal: Annual, with sterile-specific CE + supervisor attestation
Pharmacists – BCSCP Path
- Board Certified Sterile Compounding Pharmacist (BCSCP) via BPS
- Prepped through ASHP courses and recertification programs
- Ideal for managers and directors overseeing sterile operations
Costs & Timeline (Techs)
Getting certified isn’t just about passing an exam—it’s about following a clear, structured pathway that blends training, experience, and assessment.
Below is a step-by-step breakdown of the CSPT® journey for pharmacy technicians, including typical costs, time commitments, and what each stage requires.
Step | Action | Cost/Time |
CPhT Certification | Entry-level | Variable |
Sterile Compounding Course | 40-hour lab + lecture | Tuition-based |
Experience | 1 year supervised (or 3 yrs pathway) | Time investment |
CSPT® Exam | 75 Qs | $199 total ($50 app + $149 exam) |
Renewal | Annual sterile CE | Ongoing |
Some employers waive the $50 app fee through PTCB Direct Billing.
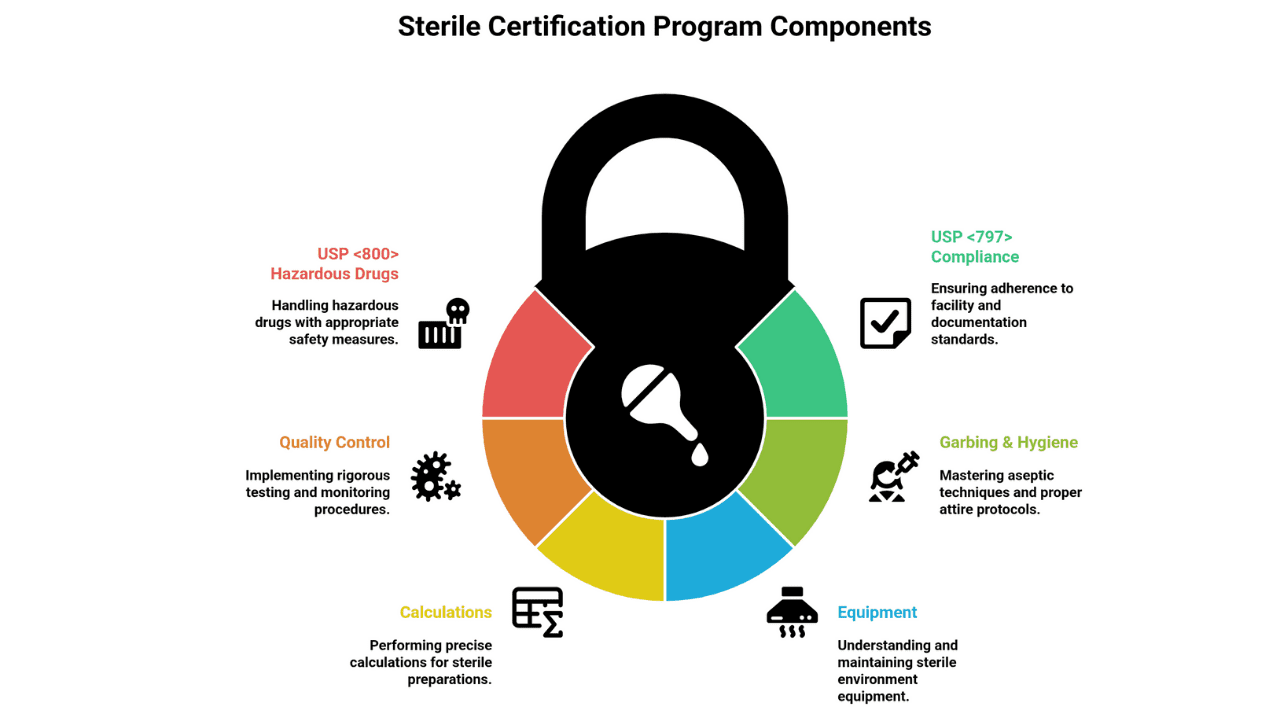
What You Learn in a Sterile Certification Program
Here’s what high-quality IV/sterile programs drill—mapped to the 2023 USP <797> (and USP <800> for HDs)—so you’re inspection-ready from day one:
- USP <797> compliance: Facility, BUDs, logs
- Garbing & hygiene: Correct gowning order, aseptic hood work
- Equipment: ISO 5 hoods, isolators, environmental monitoring
- Calculations: Dilutions, drip rates, TPN formulation
- Quality control: Media fills, gloved fingertip tests
- USP <800> Hazardous Drugs: Chemo workflows & PPE
Hands-on 40-hour sterile courses let you practice real cleanroom techniques before taking the CSPT® exam.
Career Impact & Job Outlook
With USP <797>-aligned skills and certification, here’s how that expertise translates into real roles and upward mobility across settings:
- Tech roles: IV compounding specialist, oncology technician, sterile compounding coordinator
- Pharmacist roles: Compounding manager, QA officer, pharmacy director in sterile facilities
According to the BLS, the median annual pay for pharmacy technicians in hospitals was significantly higher than in retail settings, showing the premium placed on sterile skills.
Real-World Technique Highlights
Master these non-negotiables—inspectors look for them, and they’re the small habits that keep every CSP truly sterile.
- Garbing order: Shoe covers → hair/beard cover → mask → wash hands → gown → sanitize → sterile gloves
- First-air discipline: Never block airflow to a sterile site
- Surface sampling: Weekly for Cat 3; monthly for Cat ½
- Labeling: Include patient, drug, dose, BUD, preparer/checker initials, lot numbers
Technique | Why It Matters |
Media Fill Test | Proves aseptic technique works in real time |
Gloved Fingertip Test | Detects contamination risk from hands |
Hood Disinfection | Maintains ISO 5 airflow and sterility |
Peer Double-Check | Prevents dosage miscalculations |
Beyond Certification — Growth & Advanced Roles
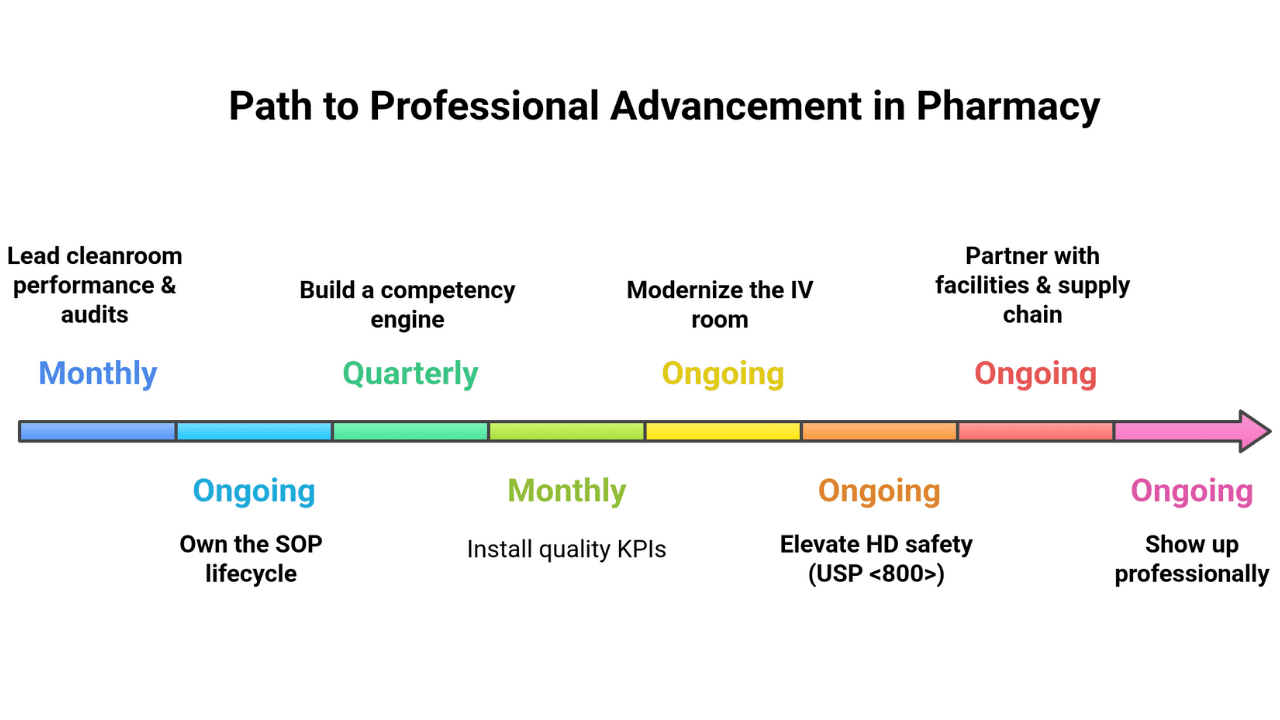
Earning the credential opens the door; what you do next determines your title, pay, and impact. Use these moves to level up fast:
- Lead cleanroom performance & audits. Run monthly surface-sampling dashboards, mock TJC/PCAB surveys, and CAPA plans; close findings within 30 days and track trend reductions.
- Own the SOP lifecycle. Rewrite workflows to Category 3 parity, implement change control/versioning, and add visual one-pagers at the hood for zero-ambiguity steps.
- Build a competency engine. Schedule q6-month (Cat 1/2) and q3-month (Cat 3) validations, standardize media fill + gloved fingertip checklists, and target >95% pass rates.
- Install quality KPIs. Monitor action-level excursions, CFU trends, batch failure rate, labeling errors, and BUD deviations; publish a one-page KPI report to leadership monthly.
- Modernize the IV room. Champion gravimetric verification, barcode checks, CSTD protocols, and (where viable) compounding robots; document ROI in error reduction and time saved.
- Elevate HD safety (USP <800>). Audit segregation, PPE compliance, spill drills, and HD waste streams; coach the team to “first-time-right” behavior.
- Partner with facilities & supply chain. Validate pressure differentials, filter changes, calibration logs, and sterile supply receipt workflows that won’t contaminate your buffer room.
- Claim the titles. Tech tracks: Lead IV Technician, Compounding Coordinator, QA Specialist, Cleanroom Supervisor. Pharmacist tracks: BCSCP → Compounding Manager, QA Pharmacist, PIC, Director of Sterile Products.
- Show up professionally. Present at P&T/CQI committees, share quarterly learnings, join ASHP/NPTA communities, and stack targeted CE toward leadership goals.
The horizon: Automation, closed-system transfer devices, advanced biologics/ATMPs, and data-driven QA are reshaping sterile compounding. Get in early, measure relentlessly, and lead the change.
Conclusion
Sterile compounding is where pharmacy precision meets life-or-death safety. The 2023 USP <797> updates made certification the baseline for safe practice. Whether you’re a pharmacy technician or pharmacist, earning a sterile compounding credential future-proofs your career, reassures employers, and most importantly—protects patients.
- Become IV-Certified in 40 Hours
Enroll in our Sterile Compounding (IV) Certification—hands-on, NPTA-approved, built to USP <797> standards. Gain aseptic confidence, career leverage, and compliance security. [Sign Up Today!]
Program Offered
- Pharmacy Technician Training
- Online Medical Assistant
- Medical Billing and Coding Specialist Program
- Cloud Computing Technician Training
- Computer Network Technician
- Business and Accounting
- Radiology Technician Training
- Medical Assistant Program
- Computer Support Technician
- Cybersecurity Program
- Virtual Assistant Training
This article is written by
Share this article
Program Offered
- Pharmacy Technician Training
- Online Medical Assistant
- Medical Billing and Coding Specialist Program
- Cloud Computing Technician Training
- Computer Network Technician
- Business and Accounting
- Radiology Technician Training
- Medical Assistant Program
- Computer Support Technician
- Cybersecurity Program
- Virtual Assistant Training
This article is written by
Share this article
Frequently Asked Questions FAQ's
What are compounded sterile preparations (CSPs)?
They are sterile meds (IVs, injections, eye drops, irrigations) prepared under USP <797> standards in cleanrooms or sterile hoods.
Is IV compounding the same as sterile compounding?
IV compounding is a subset of sterile compounding—most sterile work involves IVs, but it also covers ophthalmics and spinal injections.
Who can perform sterile compounding?
Pharmacists and trained technicians. Many states (e.g., Texas) require a 40-hour sterile compounding course + documented competency.
What changed in USP 2023?
Introduced Categories 1–3, stricter BUDs, more frequent competency testing, and tighter environmental monitoring rules.
How much does CSPT® cost, and how long does it take?
$199 (application + exam). Timeline: CPhT → 40-hour training → 1 year experience → CSPT exam → annual renewal.
Are there pharmacist-level sterile compounding credentials?
Yes—BCSCP (Board Certified Sterile Compounding Pharmacist) is the advanced credential, with prep and recertification offered by ASHP.
Related Articles






CCI Training Center Proudly Completes
41 Years in Career Training Services